Abstract
Background: Novel therapies for multiply relapsed follicular lymphoma (FL) are often evaluated in single arm trials with no comparative data on patients receiving usual care. This study (ReCORD-FL) therefore sought to construct a historical control cohort to augment current and future single arm trials in relapsed/refractory (r/r) FL. The analytic aims were to document patient characteristics, treatment patterns and clinical outcomes in a r/r FL population treated with standard therapies in routine practice.
Methods: This was a retrospective cohort study via medical record review in 10 oncology centers across North America and Europe. Adult patients were required to meet several criteria defining multiply r/r FL (i.e., r/r after ≥2 lines of therapy, or relapsed during or within 6 months after completion of anti-CD20 antibody maintenance, or relapsed after autologous HSCT). Patients were also required to have ≥1 line of systemic therapy (i.e., first qualifying salvage therapy) after first meeting the r/r FL criteria; the date of first qualifying salvage therapy defined the study index date. At index, patients were required to have grade 1-3A FL, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and no evidence of prior histological transformation. Outcomes were observed from the index date and from the start of each therapy line until the earliest of death, last follow-up, or December 31, 2020 (data cutoff). Endpoints were complete response (CR) rate, overall response rate (ORR), time to next treatment (TTNT), progression-free survival (PFS), and overall survival (OS). In a subgroup analysis, endpoints were examined by double refractoriness (r/r to both an anti-CD20 mAb and an alkylator) and POD24 status at index (best response of stable/progressive disease or relapsed within 24 months of front-line anti-CD20 mAb-containing therapy). Time to event outcomes were analyzed using the Kaplan-Meier method.
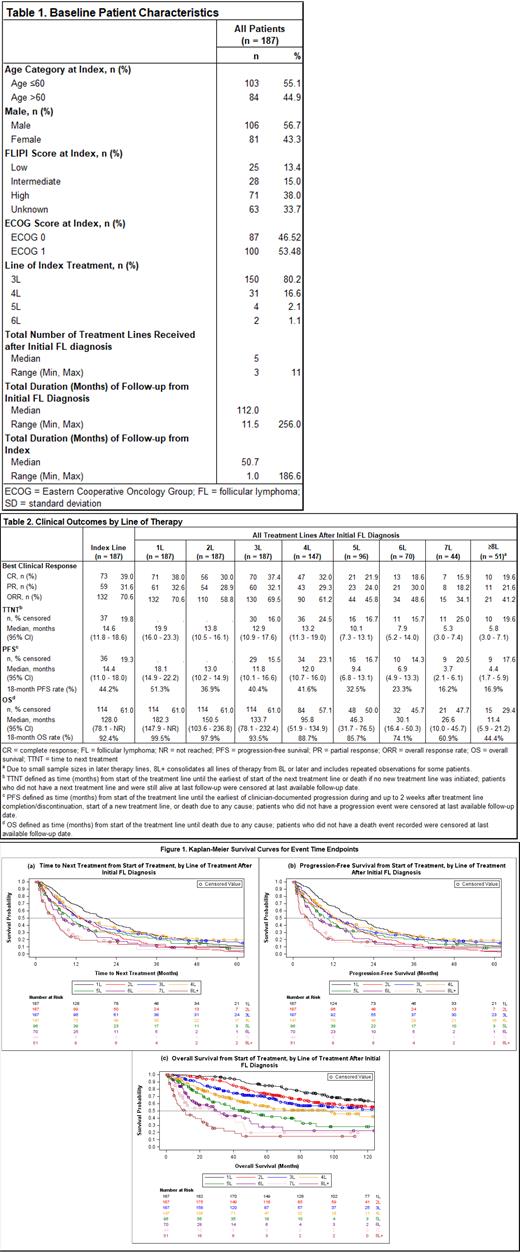
Results: A total of 187 patients were identified for inclusion (Table 1). Most patients' (80.2%) first qualifying salvage (index) therapy occurred in third line (3L) (range: 3L - 6L). Anti-CD20 mAb plus chemotherapy (including alkylating and/or non-alkylating agents) was the most common index regimen (64.2% of patients); 8% received anti-CD20 mAb monotherapy, 11.2% received alkylator-based chemotherapy alone (i.e., an alkylator-containing regimen without anti-CD20 mAb), and 16.6% received other therapies (i.e., other regimens containing neither anti-CD20 mAb nor alkylator). Median follow-up from FL diagnosis was 9 years (range: 1 - 21 years), over which a median of 5 (range: 3 - 11) lines of therapy were observed per patient. CR rate and ORR to the index treatment were 39.0% and 70.6%, respectively (Table 2). Median (95% confidence interval [CI]) TTNT and PFS from index were 14.4 (11.8 - 18.6) and 14.6 (11.0 - 18.0) months, respectively; median OS from index was 128 months (10.6 years). Compared with non-double refractory, those with double refractory disease at index had numerically lower CR (34.3% vs. 45.1%) and ORR (67.6% vs. 74.4%) and substantially shorter median (95% CI) TTNT (11.8 [9.0 - 15.2] vs. 20.9 [14.4 - 26.2] months), PFS (10.7 [7.7 - 14.5] vs. 20.1 [14.4 - 25.4] months), and OS (78.1 [45.8 - 146.7] vs. [Not Reached] months). Outcomes were similarly less favorable for patients who were POD24 at index. All outcomes steadily worsened across successive treatment lines (Table 2, Figures 1a-c). ORR, for example, decreased from 69.5% in 3L (n = 187) to 45.8% in 5L (n = 96) and 41.2% in ≥8L (n = 51), while median (95% CI) PFS decreased from 11.8 (10.1 - 16.6) months in 3L to 9.4 (6.8 - 13.1) months in 5L and 4.4 (1.7 - 5.9) months in ≥8L; median (95% CI) OS had a similar trend: 133.7 (78.1 - 232.4), 46.3 (31.7 - 76.5), and 11.4 (5.9 - 21.2) months in 3L, 5L, and ≥8L, respectively.
Conclusions: Our findings further demonstrate the poor outcomes and limited survival in FL patients with multiply r/r, double refractory, or POD24 disease. In assessing response rates and PFS, it is important to consider that response assessment criteria and schedules were more heterogenous in the routine practice settings of ReCORD-FL than in clinical trials. Based on comparability of results with another similar study (SCHOLAR-5), the robustness of data collected, and continued low likelihood of randomized trials in this area, ReCORD-FL provides valuable historical control data for new r/r FL therapies in development.
Salles: Beigene, BMS/Celgene, Debiopharm, Genentech/Roche, Genmab, Incyte, Ipsen, anssen, Novartis. Kite/Gilead, Loxo, Miltneiy, Rapt, TAKEDA, Velosbio, Allogene: Consultancy; Abbvie, Epizyme, Morphosys, Regeneron: Consultancy, Honoraria; Bayer: Honoraria. Schuster: Abbvie: Consultancy, Research Funding; Acerta Pharma/AstraZeneca: Consultancy; Alimera Sciences: Consultancy; BeiGene: Consultancy; Juno Theraputics: Consultancy, Research Funding; Loxo Oncology: Consultancy; Tessa Theraputics: Consultancy; Genentech/Roche: Consultancy, Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Adaptive Biotechnologies: Research Funding; Incyte: Research Funding; TG Theraputics: Research Funding; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Nordic Nanovector: Consultancy; Celgene: Consultancy, Honoraria, Research Funding. Kuruvilla: Seattle Genetics: Honoraria; Merck: Honoraria; Novartis: Honoraria; Gilead: Honoraria; BMS: Honoraria; Antengene: Honoraria; Amgen: Honoraria; AbbVie: Honoraria; Karyopharm: Honoraria, Other: Data and Safety Monitoring Board; Janssen: Honoraria, Research Funding; Incyte: Honoraria; Medison Ventures: Honoraria; Roche: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Pfizer: Honoraria; TG Therapeutics: Honoraria. Patten: GILEAD SCIENCES: Honoraria, Research Funding; ROCHE: Research Funding; ASTRA ZENECA: Honoraria; NOVARTIS: Honoraria; JANSSEN: Honoraria; ABBVIE: Honoraria. von Tresckow: Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other, Research Funding; Pentixafarm: Consultancy, Honoraria; AbbVie: Other: congress and travel support; BMS-Celgene: Consultancy, Honoraria, Other: congress and travel support; AstraZeneca: Honoraria, Other: congress and travel support; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Kite-Gilead: Consultancy, Honoraria; MSD: Consultancy, Honoraria, Other: congress and travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: congress and travel support, Research Funding. Smith: Alexion, AstraZeneca Rare Disease: Other: Study investigator; Celgene, Genetech, AbbVie: Consultancy. Davis: Novartis, Vertex Pharmaceuticals, Pfizer, Eisai, Eli Lilly, AstraZeneca: Research Funding. Nagar: Novartis, AstraZeneca, Eisai: Research Funding. Zhang: Novartis: Current Employment, Current equity holder in publicly-traded company. Bollu: Novartis: Current Employment, Current equity holder in publicly-traded company. Jousseaume: Novartis: Current Employment, Current equity holder in publicly-traded company. Ramos: Novartis: Current Employment, Current equity holder in publicly-traded company. Wang: Eli Lilly: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; InnoCare: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding. Link: Genentech/Roche: Consultancy, Research Funding; MEI: Consultancy; Novartis, Jannsen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal